Background and Significance: Hypereosinophilic syndrome (HES) is a rare, heterogeneous disorder defined by blood eosinophilia (absolute eosinophil count [AEC] >1500 cells/μL) on 2 examinations ≥1 month apart and eosinophil-mediated end-organ involvement, including dermatologic, pulmonary, gastrointestinal, and/or cardiovascular manifestations. Symptoms and health-related quality of life vary depending on the organ systems involved. HES is often treated with corticosteroids and other immunosuppressants. However, long-term use of these agents is associated with decreased efficacy and significant toxicity. Whereas anti-IL-5 antibody mepolizumab was recently approved for the treatment of HES (Roufosse, et al. J Allergy Clin Immunol. 2020;146(6):1397-1405), response is not universal and additional targeted agents are clearly warranted. Benralizumab is a humanized, afucosylated, anti-interleukin-5 receptor α monoclonal antibody that induces rapid, near-complete depletion of eosinophils through antibody-dependent, cell-mediated cytotoxicity. It is currently approved for use in patients with severe eosinophilic asthma and has demonstrated AEC-reducing effects in patients with HES (Kuang, et al. N Engl J Med. 2019;380(14):1336-46).
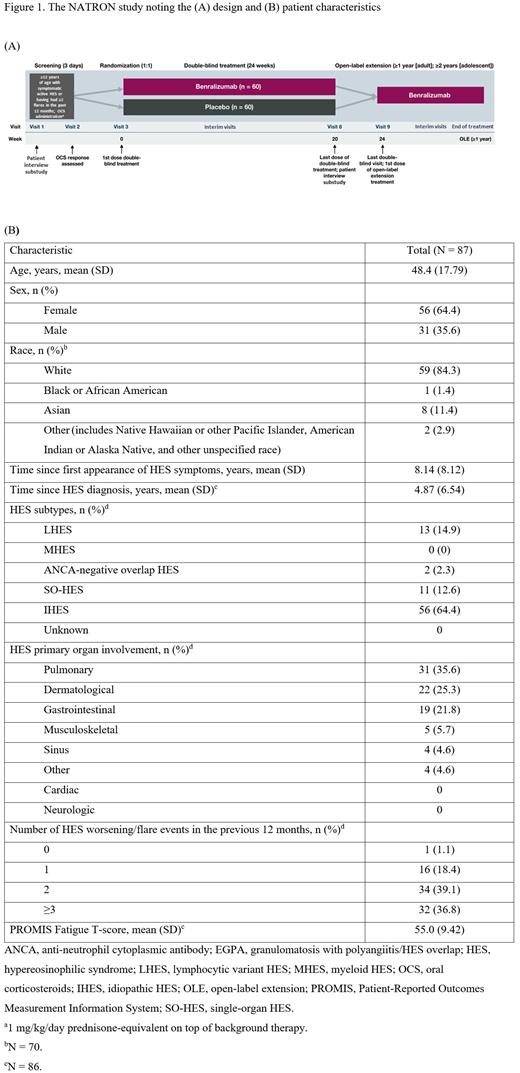
Study Design and Methods:This phase 3, multicenter, randomized, double-blind (DB), placebo-controlled study (NATRON; NCT04191304) evaluating the efficacy of benralizumab versus placebo will enroll ~120 patients with documented HES (Figure 1A). Recruitment will stop after approximately 38 patients have had their first HES worsening/flare during the DB period, at which point the data cutoff for the primary analysis will occur. Eligible patients are ≥12 years of age with an AEC ≥1000 cells/mL at screening, have active signs/symptoms of an HES worsening/flare or a history of ≥2 HES worsening/flares within 12 months before enrollment, and have a stable treatment regimen (≥4 weeks). Patients must be FIP1L1-PDGFRA-negative and corticosteroid-responsive, defined as an AEC of <1000 cells/μL after 2 days of corticosteroid treatment. The primary endpoint is time to first HES worsening/flare during the DB period. Key secondary endpoints during the DB period include the proportion of patients who experience HES worsening/flare events, the number of HES worsening/flare events (annualized rate/year), time to first hematologic relapse, and change from baseline in fatigue at week 24 as assessed by the Patient-Reported Outcomes Measurement Information System (PROMIS) Fatigue Short Form 7a. Patients who complete the 24-week DB treatment period will continue with active treatment in a ≥52-week open-label extension. We present the study design and interim baseline characteristics of patients recruited to date.
Results:As of the June 6, 2023, cutoff date, 87 patients were enrolled in the full analysis set (Figure 1B). Among these patients, the mean (SD) age was 48.4 (17.79) years, 64.4% were female, and 84.3% were White. The mean (SD) time since the first appearance of HES symptoms and since HES diagnosis was 8.14 (8.12) and 4.87 (6.54) years, respectively. The proportion of patients with the lymphocytic variant HES was 14.9%. The most common primary organs involved were the lungs (35.6%), followed by the skin (25.3%) and the gastrointestinal tract (21.8%). Thirty-four (39.1%) and 32 (36.8%) patients experienced 2 and ≥3 HES worsening/flare events in the previous 12 months, respectively. The mean (SD) PROMIS Fatigue T-score was 55.0 (9.42). Mean (SD) baseline eosinophil levels were 1960 (2610) cells/µL, with median (interquartile range) levels of 1120 (60, 20910) cells/µL.
Conclusions: Results from the NATRON study will provide insight into whether benralizumab is beneficial in addition to background therapy in patients with FIP1L1-PDGFRA-negative steroid-responsive HES.
Funding: This study is funded by AstraZeneca (Gothenburg, Sweden). This work is funded in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Acknowledgments:Vivian H. Shih, Dianne Griffis, Himanshu Parikh, Ron A. Chen, Ying Fan, Gaëll Mayer, Rohit Katial, and Ioannis Psallidas.
Disclosures
Akuthota:Vindico: Honoraria; Rockpointe: Honoraria; Prime CME: Honoraria; AKH: Honoraria; Medscape/WebMD: Honoraria; UpToDate: Patents & Royalties; American Partnership for Eosinophilic Disorders: Other: grant support; National Institutes of Health (USA): Other: grant support ; Regeneron: Research Funding; Sanofi: Consultancy, Research Funding; GlaxoSmithKline: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding. Klion:UpToDate: Patents & Royalties. Ogbogu:AstraZeneca: Consultancy; GlaxoSmithKline: Consultancy; Blueprint Medical: Research Funding. Roufosse:UpToDate: Patents & Royalties; GlaxoSmithKline: Consultancy, Other: Speaker fees ; AstraZeneca: Consultancy, Other: Speaker fees; Menarini: Other: Speaker fees; Merck: Consultancy. Bahadori:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Bouma:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Brooks:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Keith:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Ho:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Jain:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Datto:AstraZeneca: Current Employment, Current equity holder in publicly-traded company.